How Long Should Iv Site Be Sore
Note: This guideline is currently nether review.
Introduction
Aim
Definition of terms
Assessment
Management
Companion Documents
References
Show Table
Introduction
Peripheral intravenous catheters (PIVC) are the most commonly used intravenous device in hospitalised patients. They are primarily used for therapeutic purposes such as administration of medications, fluids and/or claret products as well as claret sampling.
Aim
The aim of this guideline is to provide an outline of the ongoing maintenance and management of the PIVC for patients in hospital, outpatient, and home healthcare settings. For information related to insertion of PIVC, delight refer to intravenous access guideline . Nurses who are deemed competent in IV insertion could proceed to insert PIVC in consultation with NUM/CSN'southward.
Definition of terms
- Peripheral 4 devices: are cannula/catheter inserted into a small peripheral vein for therapeutic purposes such as administration of medications, fluids and/or claret products.
- Aseptic technique: is a part of all procedures which aims to prevent pathogenic microorganisms, in sufficient quantity to cause infection, from being introduced to susceptible primal sites by key parts, hands, surfaces and equipment. Therefore, different sterile techniques, standard and surgical aseptic techniques are possible and tin can exist achieved in typical hospital and community settings.
- Decontaminate hands: Perform paw hygiene in club to protect the patient from organisms which may enter their fundamental sites or devices during a procedure.
- Key Parts: role of the device/due south that must remain aseptic throughout the clinical procedures. Examples of Primal parts include, catheter hub, needleless connector, syringe hub, needle etc.
- Key Sites: the area on the patient such equally 4 insertion site that must exist protected from microorganisms.
- Extravasation: An extravasation occurs when at that place is accidental infiltration of a vesicant drug or fluid into the tissue surrounding the venipuncture site.
- Infiltration: occurs when drugs or fluid infiltrates into the tissue surrounding the venipuncture site. This happens when the tip of catheter slips out of the vein, catheter passes through the wall of the vein, or as blood vessel wall stretches which allows fluid to infuse into the surrounding tissue.
- Phlebitis: a sign of vessel damage. The cause can exist chemical (due to the osmolarity of the solution), mechanical (from trauma at insertion or movement) or infective (microorganisms contaminating the device). Signs include swelling, redness, heat, induration, purulence, a palpable venous cord (difficult vein) and pain related to local inflammation of the vein at or near the insertion site.
- Infusion Pump: refers to infusions pumps similar big volume pumps (LVPs)/volumetric pumps east.g. Alaris Signature Edition (SE), Syringe drivers (e.g. Alaris GH+), Patient Controlled Analgesia/PCA pumps (Alaris PCAM) etc.
- Double checking:refers to the practice of two clinicians (appropriately endorsed Enrolled nurses (EN), Registered Nurses (RN), Doctors or Pharmacists) independently checking the medications.
Assessment
Patient and IV site assessments should exist done on a regular basis.
PIVC assessment includes:
- Assessment of PIVC insertion site - Catheter position, patency/occlusion, limb symmetry, whatever signs of phlebitis (erythema, tenderness, swelling, pain etc.), infiltration/extravasation. PIVC are considered equally high risk for force per unit area injury. PIVC sites should be checked hourly for force per unit area sore and any signs of infection unless documented otherwise. http://www.rch.org.au/rchcpg/hospital_clinical_guideline_index/Pressure_Injury_Prevention_and_Management/.
- Assessment of PIVC dressing and splints: check securement of dressing- if information technology's intact, make clean and dry or if information technology's loose or if visible ooze was present underneath the dressing. Check splint tapes are not too tight or restrictive.
- Assessment of 4 lines, equipment and 4 fluid infusions:
- If the patient is receiving continuous Four fluid infusion- observations of the Four site, blazon of fluid and volume infused, accurate rate of infusion for patient and pressure alarms of infusion pumps are observed hourly and documented in the fluid balance flowsheet.
- If the patient (inpatient setting) is having intermittent infusion, 8 hourly assessments are a minimum. Unstable patients who have signs and symptoms of complications are to exist assessed more than ofttimes.
- If the patient no longer requires 4 admission for infusions, remove the cannula at the earliest to avoid complications.
- If the patient is receiving continuous Four fluid infusion- observations of the Four site, blazon of fluid and volume infused, accurate rate of infusion for patient and pressure alarms of infusion pumps are observed hourly and documented in the fluid balance flowsheet.
- For Hospital in the Home (HITH) patients, the nurse will assess the PIVC with each visit.
- Caregiver and patient pedagogy will exist provided on the signs of injuries and the procedure of contacting the nurse.
Direction
Assistants of intravenous fluid, drug infusions or blood products
a) Continuous infusion of IV fluids
Cess and documentation of findings are to be completed hourly to make up one's mind effective delivery of prescribed medications and fluid.
- Each bag of fluid is independently double checked and a signed patient characterization is put on the bag.
- Check the solution is the prescribed 1, the rate of infusion, and the amount infused is noted.
- Document the infused volume: Hourly on fluid balance flowsheet (it is advised to clear the infusion pump hourly)
- Check the infusion site for any signs of complications and certificate the assessment findings hourly in fluid balance flowsheet
- Review the cumulative book infused and fluid output as required based on patient'due south clinical status.
- Pump pressures for each IV line should be documented hourly or when adjusted on the flow sail
Infusion Pump Force per unit area
Pressure limit defaults for intravascular infusion pumps are programmed past Biomedical Engineering, based on the manufacturer'due south recommendations.
Upper limit infusion pump pressure can exist manually increased with clinical discretion to suit:
- Increased viscosity of the fluid being administered
- High rate of the fluid being administered
- Reduced bore of the intravascular catheter
- Increased length of the intravascular catheter
- Increased level of patient action
If pump pressure exceeds the recommended limits, check the patency of the PIVC.
b) Administration of bolus/loading doses:
Administering drugs:
Drugs administered via PIVC may exist
- diluted into a bag of IV fluids
- added to the burette of an infusion set
- prepared for assistants via a volumetric infusion pump
- in a syringe for employ in a syringe driver
- administered straight as a bolus or push
The nigh appropriate method should exist selected depending on book of diluent required, patient condition, fluid residual and intended charge per unit of delivery.
Drugs administered via:
- Burette of an infusion set: to dilute the drug in a smaller volume via burette giving organization, hang the bag of infusion fluid and gradually open the roller camp to permit advisable amount of diluent into the burette. Inject the prescribed drug into the burette via the additive port.
- Syringe driver: is recommended for children weighing less than 10 kg. Draw up required volume of diluent in appropriate size syringe and then pull back the syringe plunger to enable you lot to inject the drug into the syringe using aseptic technique.
- Infusion purse: Make clean the access port with disinfectant swab before injecting prepared drug into infusion fluid bag via the condiment port. Without contaminating the primal part (spike) insert the fasten on the administration set into the septum of the infusion purse.
Attach a completed drug characterization detailing the drug, dose, diluent, volume of diluent, date, time and signature of the nurse and the staff who double checked.
Access PIVC only later on cleaning the admission port and scrub the hub.
For intermittent infusions, Iv lines which are disconnected are to be discarded between infusions. Ensure the cannula is flushed with normal saline once the giving prepare is asunder from the cannula. For Opioid infusion bolus refer to the specific guidelines: Children's Pain Management Service (CPMS)(opioid infusion guideline)
Administering blood products:
- Bank check patient and blood production identification every bit per the Blood Product Transfusion Procedure.
- Administer claret production transfusions via a volumetric infusion pump or syringe driver to ensure accurate delivery. Use gravity sets only when rapid administration is required with diligent monitoring of volume.
- Use a Neonatal transfusion set (includes a 170 to 200 micron filter required for blood products) and syringe driver for delivering modest volumes of blood products.
- Using hygienic non touch on technique, spike the blood production septum with the Neonatal transfusion set and attach an appropriate sized syringe for the transfusion to the 3 fashion tap.
- Describe the required book into the syringe and prime number the rest of the neonatal transfusion set. Characterization the syringe with both patient and blood product identification details including decease date and fourth dimension of blood production.
- If rapid transfusion of pocket-size volumes is required, draw the required book into a syringe through a 170 to 200 micron filter.
- Burettes should not exist used for transfusion of blood products.
Flushing of PIVC'south
- If the cannula is accessed intermittently for the administration of medications or fluids, the cannula should be flushed prior to infusion or at least once a shift.
- Sterile 0.9% sodium chloride for injection should exist used to flush a catheter. This must be prescribed as a medication.
- The optimal volume used for intermittent injections or infusions is unclear. The literature suggests the volume of flush should equal at least twice the volume of the catheter and add on devices and a minimum of 2mL normal saline flush is recommended.
- Employ 10ml syringe for flushing to avoid excessive pressure and catheter rupture. Syringes with an internal diameter smaller than that of a 10mL syringe can produce higher pressure in the lumen and rupture the catheter. If resistance is felt during flushing and force is applied this may effect in extravasation
- Use aseptic non touch techniques including cleaning the access port (scrub the hub) with a dual disinfectant agent (e.thou. chlorhexidine and alcohol) vigorously for at to the lowest degree 15 seconds and allowing to dry prior to accessing the system.
- Flush in a pulsatile (push-break) motion.
Flush catheters:
- Immediately after placement
- Prior to and after fluid infusion (equally an empty fluid container lacks infusion pressure and will allow claret reflux into the catheter lumen from normal venous pressure level) or injection.
- Prior to and after blood drawing.
- Immediately after placement
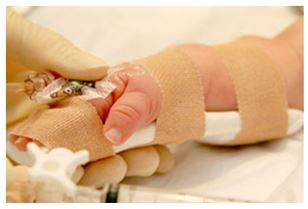
Change of PIVC dressing and securement of cannula:
- Dressings to PIVC sites are the first line of defence against infection and dislodgements. The dressing must be kept secure, make clean dry out and intact.
- Indications for dressing alter: when it becomes insecure or if at that place is blood or fluid leakage under the dressing.
- Determine the need for an assistant because patient age, developmental level and family participation prior to the procedure.
- If patient is allergic to transparent moving picture dressings, use sterile pic dressing to be used and changed daily.
- Advisedly remove the old dressing, holding the cannula in identify at all times
- Take the opportunity to thoroughly inspect the site of entry of the cannula for any sign of infection.
- Skin preparation using booze in 2% chlorhexidine is the preferred solution for dressings.
- Cleanse the area around the catheter insertion site including under the hub using a blueprint which will ensure unabridged surface area is covered.
- Allow peel preparation to air dry out prior to applying any dressing, this allows the disinfectant to piece of work.
- Consider placing a small slice of sterile cotton fiber ball or gauze underneath the hub of the cannula to reduce pressure.
- If desired, place sterile tape over the hub of the device before placing the transparent dressing.
- Cover the cannula insertion site with sterile transparent semipermeable, occlusive dressing (due east.g. Tegadermtm, Four 3000tm) placed using an aseptic non touch technique over the catheter. This volition allow continuous observation of the site and to assist stabilise and secure the catheter.
- Four board / splints are recommended to secure PIVC placed in or adjacent to areas of flexion. This volition adequately immobilize the joint and minimise the risk of venous harm resulting from flexion.
- When using Splints, ensure these are positioned and strapped with the limb and digits in a neutral position to forestall injury from restricting blood or nervus supply and to prevent pressure sores
- Inspect the splint at least daily and change if soiled past claret or fluid leakage.
- Embrace with non-compression tubular bandage. Ensure at that place is a clear window where the cannula enters the pare- insertion site, so the site can be regularly viewed.
- In Summary, when dressing a peripheral IV cannula ensure:
- it is secure
- the site is visible
- the child tin can't injure themselves, or be injured by the connections
- the kid tin can't remove or dislodge the cannula
- tapes are not too tight or restrictive.
- it is secure
- Refer to Intravenous admission–Peripheral guideline for steps involved in accessing and securing the cannula http://www.rch.org.au/clinicalguide/guidelineindex/Intravenous_access_Peripheral/
- Documentation shall contain information on the insertion site, guess of the needle and date and time of insertion has been documented in the EMR- LDA properties.
Change of Extension sets
- Extension sets are to exist changed when the access device is changed or immediately upon suspected contagion or when any intermission in integrity.
- Extension sets are to be primed and attached to the cannula at the time of 4 insertion using an aseptic non touch on technique
- When exiting the flushing of extension set you must utilise a positive pressure clamping technique.
- When non in use, extension sets must be clamped
IV Fluid Considerations via Peripheral IV line
Which Fluids and how much fluids to use
Refer to the Intravenous Fluids Clinical Practice Guideline: Intravenous Fluids
- Administering fluids containing glucose concentration greater than 12.five% will require central venous line access.
Labeling infusions:
- Label the fluid bag/syringe with engagement, time, patient name and signature of two checking staff. The label must be placed on the front end of the fluid bag ensuring the fluid proper noun, batch number, decease date and graduations remain visible (link to national standard). Labels on syringes should be placed parallel to the long axis of the syringe butt with the top edge of the label affluent with (just not covering) the graduations ( link to national standard).
- Label IV line if multiple lines are running: label close to the fluid bag or syringe or beneath the drip bedroom.
- If additives are added to infusion, please label the handbag or syringe driver with additives added.
- Canonical label can be generated by the EMR.
Fluid purse and infusion changes:
- Fluid numberless and syringes with zero additives are changed at to the lowest degree every 7 days.
- Fluid bags and infusions with additives are changed every 24 hours.
- Fresh blood products and lipid containing solutions; both the bag, syringe, giving set and lines should be removed or changed at determination of infusion or at least every 24 hours.
Line changes
- Infusion lines are replaced at least every seven days using standard aseptic technique.
- Assistants sets that have been disconnected (either accidentally or planned) are no longer sterile and to be discarded and replaced.
- If using fresh blood or fresh claret products supersede line(s) at the cease of the infusion.
- If lipid emulsion is existence infused modify the lipid syringe/bag and line every 24 hours.
Table 1.Irresolute IV bags and lines
| Bag change | 4 line change | |
| No additives in infusion | Every 7 days | Every seven days |
| Additives in infusion | Every 24 hours | Every 7 days |
| Lipid or lipid containing parenteral nutrition | Every 24 hours | Every 24 hours |
| Claret products | Every 4 hours | Upwardly to 12 hours |
Removal of PIVCs:
There is no evidence for routine replacement of PIVC unless clinically indicated. PIVC'southward should be maintained with regular assessment and documentation of complications.
The possible reasons for removal of PIVC's include a number of complications which range from infiltration, extravasation, phlebitis, occlusion, dislodgement and migration. Once the kid's treatment is over, the PIVC should exist removed to avoid whatever boosted complications.
- Perform hand hygiene
- Gear up patient and caregiver
- Perform manus hygiene and utilise non-sterile gloves, carefully remove the adhesive dressing, holding the cannula in identify at all times
- Hold a piece of sterile gauze or cotton over the exit site simply exercise non utilize pressure level
- Slowly withdraw the cannula, maintaining a neutral angle with the child'southward skin
- Cover site with dressing e.g. force per unit area dot, cotton wool and tape or Ring-Aidtm
- Advise the child and family that the cotton wool and tape or Band-Help should remain in situ for upward to 24 hours
- Remove gloves, perform mitt hygiene
- Dispose of waste according to clinical do, perform hand hygiene
- Document date and reason for removal. Ensure the device is also removed from the LDA in EMR.
Management of complications
At that place are a range of complications that could occur with the presence of a PIVC in insitu. Some of these complications can be prevented by the correct utilise of aseptic technique for insertion and maintenance equally well as assessing the device as indicated.
Common complications are:
- Infection:
- Skin-based bacteria may enter through insertion site
- Local cellulitis or systemic bacteraemia are possible.
- Phlebitis: Vein irritation
- Due to the presence of the catheter/fluids or medication
- Chronically ill patients requiring multiple and recurrent IV access.
- Infiltration/Extravasation: delivery of fluids or medications into surrounding tissue
If Infiltration/extravasation occurs... (Link to neonatal extravasation guideline).
- Immediately finish the infusion and disconnect the tubing as close to the catheter hub as possible.
- Remove the catheter without placing pressure on the site.
- Drag the affected limb.
- Apply either ice packs or warm compresses to the affected area, depending on the drug that extravasated.
- Continue to assess and document the appearance of the site and associated signs and symptoms. Some signs, such as erythema and ulceration, may be delayed for 48 hours or more after the extravasation.
- For neonatal extravasation refer to RCH guideline Neonatal Extravasation
- Plastics team to review the patient
- Certificate the engagement and fourth dimension of the infusion when extravasation was noted, the type and size of catheter, the drug administered, the estimated amount of extravasated solution, and the administration technique used.
- Certificate the patient'due south signs and symptoms, treatment, and response to treatment. Include the fourth dimension you notified the patient'due south primary care provider and the chief care provider's name.
- Immediately finish the infusion and disconnect the tubing as close to the catheter hub as possible.
Companion Documents
- http://www.rch.org.au/clinicalguide/guideline_index/Intravenous_access_Peripheral/
- http://www.rch.org.au/policy/policies/Central_Venous_Access_Device_Management/
- http://world wide web.rch.org.au/policy/policies/Medication_Management/
- http://www.rch.org.au/policy/policies/Procedural_Pain_Management/
- http://www.rch.org.au/rchcpg/hospital_clinical_guideline_index/Neonatal_Extravasation/
- http://world wide web.rch.org.au/policy/policies/Blood_Product_Transfusion/
- http://world wide web.rch.org.au/rchcpg/hospital_clinical_guideline_index/Pressure_Injury_Prevention_and_Management/
- http://www.rch.org.au/policy/policies/Aseptic_Technique/
References
- Abolfotouh, M. A., Salam, M., Bani-Mustafa, A., White, D., & Balkhy, H. H. (2014). Prospective written report of incidence and predictors of peripheral intravenous catheter-induced complications. Therapeutics and clinical risk management, (x) 993-1001.
- Ben Abdelaziz, R., Hafsi, H., Hajji, H., Boudabous, H., Ben Chehida, A., Mrabet, A., . . . Tebib, N. (2017). Full title: peripheral venous catheter complications in children: predisposing factors in a multicenter prospective cohort study. BMC Pediatrics, 17(1), 208-208. doi: 10.1186/s12887-017-0965-y
- Callaghan, S., Copnell, B., & Johnston, 50. (2002). Comparison of 2 methods of peripheral intravenous cannula securement in the pediatric setting. Periodical Of Infusion Nursing, 25(iv), 256-264.
- Fidler, H. (2010). To splint or not to splint: securing the peripheral intravenous cannula. Advances In Neonatal Care (Elsevier Science), 10(4), 204-205
- Gabriel, J. (2010). Vascular access devices: securement and dressings. Nursing Standard (Royal College Of Nursing (Great britain): 1987), 24(52), 41-46.
- Gunes, Aynur and Bramhagen, Ann-Cathrine (2018). Heparin or Sodium Chloride for Prolonging Peripheral Intravenous Catheter Apply in Children - A Systematic Review. Journal of pediatric nursing
- Hadaway, L. C. (2009). I.V. rounds. Preventing and managing peripheral extravasation. Nursing, 39(10), 26-27
- Hugill, K. (2016). Is there an optimal manner of securing peripheral Four catheters in children? British Journal of Nursing, 25(19), S20-S21. doi: x.12968/bjon.2016.25.xix.S20
- Inge J. J, A., Johanna A, H., Henriette T. K, W., Gert-Jan, v. d. Due west., Johannes Grand. M, K., & Kian D, L. (2011). Effectiveness of heparin solution versus normal saline in maintaining patency of intravenous locks in neonates: a double blind randomized controlled study. Periodical of Advanced Nursing(12), 2677. doi: 10.1111/j.1365-2648.2011.05718.x
- Keogh, S., Flynn, J., Marsh, N., Mihala, K., Davies, M., & Rickard, C. (2016). Varied flushing frequency and volume to prevent peripheral intravenous catheter failure: a pilot, factorial randomised controlled trial in adult medical-surgical infirmary patients (Vol. 17).
- Laudenbach, N., Carie A, B., Klaverkamp, 50., & Hedman-Dennis, S. (2014). Peripheral Iv Stabilization and the Rate of Complications in Children: An Exploratory Study. Journal of Pediatric Nursing, 29, 348-353. doi: x.1016/j.pedn.2014.02.002
- Lim, E. Y. P., Wong, C. Y. West., Kek, Fifty. K., Suhairi, S. Southward. B. Yard., & Yip, W. K. (2018). Improving the Visibility of Intravenous (IV) Site in Pediatric Patients to Reduce IV Site Related Complications - An Evidence-based Utilization Project (Vol. 41, pp. E39-E45).
- Lucchini, A., Angelini, Southward., Losurdo, Fifty., Giuffrida, A., Vanini, S., Elli, S., . . . Fumagalli, R. (2015). [The affect of airtight system and 7 days intravascular administration prepare replacement on catheter related infections in a general intensive care unit: a before-after report]. Assistenza Infermieristica E Ricerca: AIR, 34(3), 125-133. doi: 10.1702/2038.22138
- Malyon, Lorelle & Ullman, et al. (2014). Peripheral intravenous catheter duration and failure in paediatric acute care: A prospective accomplice study. Emergency Medicine Australasia. 26. 10.1111/1742-6723.12305.Marsh, Northward., Webster, J., Mihala, G., & Rickard, C.
- M. (2015). Devices and dressings to secure peripheral venous catheters to forestall complications.
- Marsh, Due north., Webster, J., Mihala, G., & Rickard, C. M. (2015). Devices and dressings to secure peripheral venous catheters to prevent complications.
- Morris, W., & Tay, K. (2008). Strategies for preventing peripheral intravenous cannula infection. British Periodical Of Nursing, 17(19), S14-21.
- O'Grady N, Alexander M, Burns L, Dellinger East, Garland J, et al. (2011) The Healthcare Infection Control Practices Advisory Committee (HICPAC). Guidelines for the prevention of intravascular catheter-related infections. Clinical Journal of Infectious Diseases 2011 May;52(ix): 1087–99.
- Phulara, U. (2018). Effectiveness of Normal Saline Flush versus Heparin Saline Flush in Maintaining the Patency of Peripheral Intravenous Cannula and on Occurrence of Intravenous Local Vascular Complications in Patients Receiving Intermittent Intravenous Medications, 51.
- Rickard, C. G., Marsh, N., Webster, J., Runnegar, N., Larsen, E., McGrail, M. R., . . . Playford, E. G. (2018). Dressings and securements for the prevention of peripheral intravenous catheter failure in adults (Salvage): a pragmatic, randomised controlled, superiority trial (Vol. 392, pp. 419-430).
- Rickard, C. One thousand., Webster, J., Wallis, G. C., Marsh, N., McGrail, M. R., French, 5., . . . Whitby, G. (2012). Routine versus clinically indicated replacement of peripheral intravenous catheters: a randomised controlled equivalence trial. Lancet, 380(9847), 1066-1074.
- Rickard, C. M., McCann, D., Munnings, J., & McGrail, One thousand. R. (2010). Routine resite of peripheral intravenous devices every 3 days did not reduce complications compared with clinically indicated resite: a randomised controlled trial. BMC Medicine.
- Rickard, C. (2004). Prolonged utilize of intravenous administration sets: a randomised controlled trial.
- Rita, A., Hindra Irawan, S., & Pustika, A. (2013). Elapsing of peripheral intravenous catheter use and evolution of phlebitis. Paediatrica Indonesiana, Vol 53, Iss 2, Pp 117-20 (2013)(2), 117. doi: ten.14238/pi53.two.2013.117-twenty
- Smith, B., & Royer, T. I. (2007). New standards for improving peripheral i.5. catheter securement. Nursing, 37(3), 72-74.
- Tripathi, S., Kaushik, V., & Singh, V. (2008). Peripheral IVs: Factors Affecting Complications and Patency-A Randomized Controlled Trial, 182.
- Ullman, A., Marsh, N., & Rickard, C. (2017). Securement for vascular access devices: looking to the hereafter. British Journal of Nursing, 26(8), S24-S26. doi: x.12968/bjon.2017.26.8.S24
- Ullman AJ, Cooke ML, Gillies D, Marsh NM, Daud A, McGrail MR, O'Riordan E, Rickard CM. Optimal timing for intravascular administration fix replacement. Cochrane Database of Systematic Reviews 2013, Issue ix.
- Webster, J. (2015). Clinically-indicated replacement versus routine replacement of peripheral venous catheters. Cochrane Database of Systematic Reviews(8).
Evidence Table
The bear witness tabular array can exist found hither.
The development of this nursing guideline was coordinated past Mercy Thomas, Nursing Educator, and approved by the Nursing Clinical Effectiveness Committee. Updated December 2018.
Source: https://www.rch.org.au/rchcpg/hospital_clinical_guideline_index/Peripheral_Intravenous_IV_Device_Management/
Posted by: mcclurgyoughat.blogspot.com

0 Response to "How Long Should Iv Site Be Sore"
Post a Comment